A joint research team from the University of Illinois Chicago and the University of California, Davis has developed a revolutionary bio-3D printing technology capable of preparing tissue-specific high cell density bio-inks, successfully constructing complex multi-phase tissue structures. This study, published in Materials Today, achieves precise control over spatial differentiation and tissue development by combining stem cell bio-inks with gelatin microparticles loaded with growth factors.
Core Technological Breakthrough
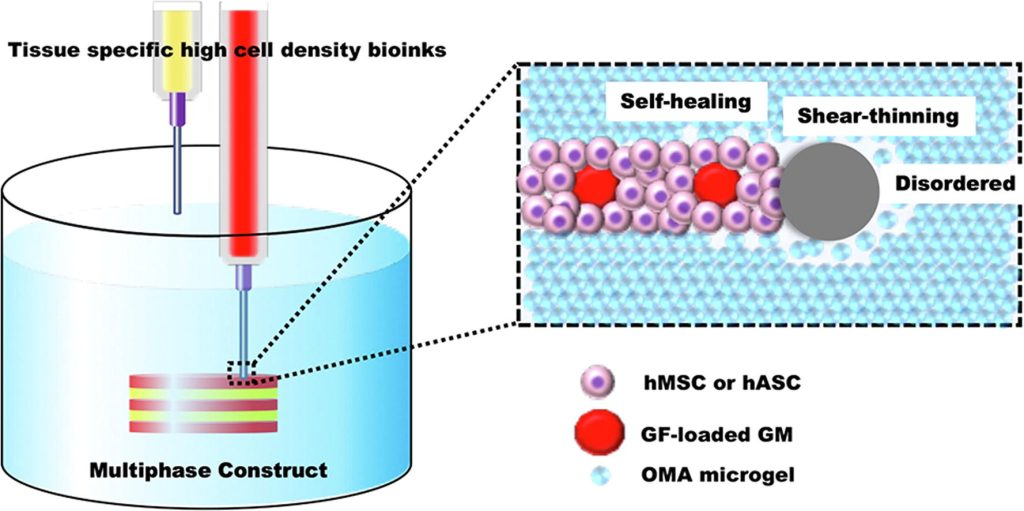
Innovative Bio-Ink System: Utilizes shear-thinning, light-crosslinked alginate microgel supporting bath to achieve high shape fidelity and adjustable degradability.
Dual-Mode Cell Carrier: Supports both single stem cells and multi-cell aggregates as the bio-ink matrix.
Precise Growth Factor Controlled Release: Achieves localized sustained biochemical signaling through gelatin microparticles loaded with TGF-β1 and BMP-2.
Self-Organizing Capability: Post-printing, cells spontaneously form multi-phase tissue structures such as cartilage-bone through aggregation.
Key Experimental Results
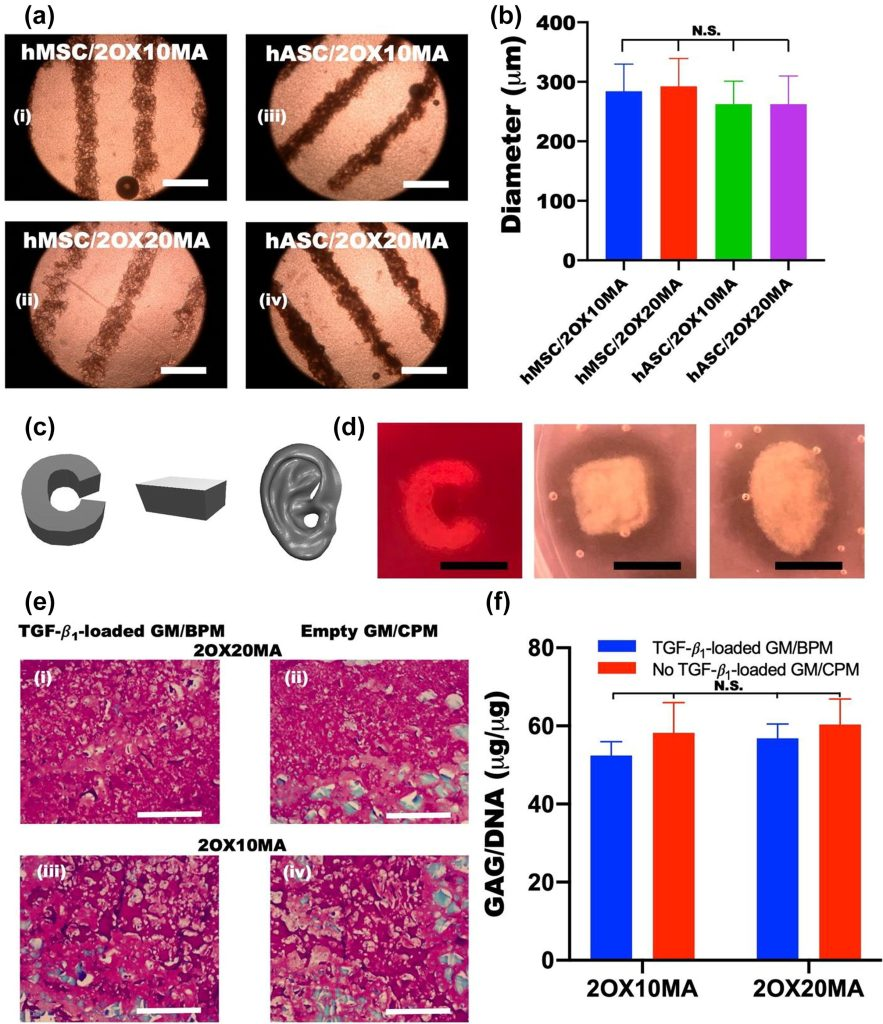
Printing Precision: Achieved fine fiber printing with a diameter of 500μm using a 22G nozzle.
Structural Stability: Maintained initial geometry and clear tissue interfaces after 4 weeks of culture.
Functional Validation: Histological analysis confirmed specific glycosaminoglycan deposition in the cartilage region and significant calcification in the bone region.
Comparison of Technical Advantages
| Traditional Bioprinting | New Technology Solution |
| Relies on biomaterial matrices, hinderingdirect cell – cell interactions | Pure cell – based ink, promotingcell self – assembly |
| Systemic administration of growth factors, lacking spatial specificity | Local controlled – release of growth factors, precisely guiding differentiation |
| Uncontrollable degradation behavior | Programmable degradation microenvironment |
Application prospects
This technological platform brings new possibilities to the following fields:
Regenerative Medicine: The ability to construct physiologically relevant bone-cartilage composite grafts opens up new avenues for tissue replacement, particularly in joint and bone repair. This can potentially address challenges in repairing complex tissue defects, offering solutions that are more functional and biologically integrated than current approaches.
Disease Modeling: The creation of pathological models that involve multiple cell types allows for more accurate simulations of diseases. This could enable researchers to better understand the progression of diseases and test potential treatments on a variety of tissues that more closely resemble human biology.
Drug Screening: Developing testing platforms that are closer to human tissue characteristics will improve the accuracy of preclinical drug screening. This approach can help identify effective drugs more efficiently while reducing reliance on animal models and improving the relevance of test results to human physiology.
The research team indicates that the next step will focus on addressing vascularization to improve tissue maturity and accelerate clinical translation. By tackling this issue, they aim to create more functional, mature tissue constructs that can better mimic the biological processes of natural tissues.
This technology, alongside recent innovations such as SCOBY cellulose scaffolds and heart-on-a-chip models, is driving the field of bio-3D printing toward more functional and complex designs, offering transformative solutions for medicine, healthcare, and biotechnology.